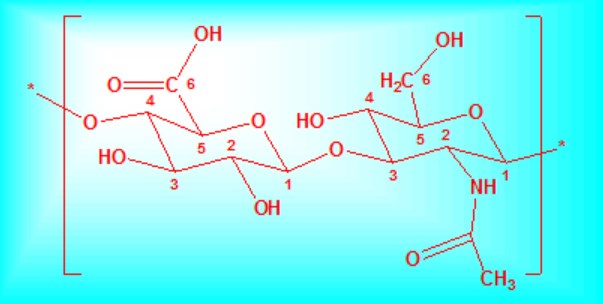
PRIMARY STRUCTURE OF HYALURONAN
Hyaluronan (HA), a naturally occurring linear polysaccharide is composed of repeating disaccharide units of D-glucuronic acid (GlcUA) and N-acetyl-D-glucosamine (GlcNAc): [-β(1,4)-GlcUA-β(1,3)-GlcNAc-]n (see Fig. 1). The precise chemical structure of HA was first determined by Bernard Weissman and Karl Meyer in 1954. To verify the structure of HA, Meyer and his associate carried out a series of chemical reactions including esterification of the disaccharide, oxidation of the glucosamine residue to glucosaminic acid, and reduction of the uronic ester to a glucose residue. About the structure of polymeric hyaluronan, it has been inferred from its stability to acid hydrolysis and other considerations that the acetyl is present as an N-acetylglucosamine residue. It demonstrated the absence of vicinal hydroxyl groups (C-1) (Fig. 1) through that HA didn’t react easily with periodate. The rotation of the polymer, [α]D -70°suggests that this linkage has the β- configuration. The regularity of the size distribution of the oligosaccharides isolated from enzymatic digests points clearly to an unbranched or nearly unbranched polymer. The primary structure of polysaccharide consists of N-acetyl-D-glucosamine and D-glucuronic acid alternately linked together through β1,3 and β1,4 glycosidic bonds (Fig. 1) 1, 2.
Fig. 1 The repeat disaccharide of HA, D-glucuronic acid and N-acetyl-glucosamine

Higher orders of structure of Hyaluronan
Along with the development of study to structures and applications of HA, the higher orders of the structure of HA were discovered, such as the secondary and tertiary structures. HA can form two stable secondary structures depending on the environment. One forms in aqueous solutions, and the other in conditions of “water shortage” (see Fig. a, b) 3.
Fig.2 Secondary structure of HA in dimethyl sulphoxide (DMSO) containing water (a) and DMSO without water (b).

The secondary structures are mainly formed as tape-like twofold helices by twisting each disaccharide unit through 180°compared with those ahead and behind it in the chain. For the secondary structure, intramolecular hydrogen bond was formed between the functional groups such as NH, OH, and C=O. These bonds make sure the polysaccharide chain is rigid. Further, the meshwork-like β-sheet tertiary structure is formed when the concentration of HA solution is higher. The helical structure is reinforced by the intermolecular hydrogen bonds between acetamido and carboxylate groups of different HA chains and interactions between hydrophobic patches (Fig. 3)4, 5.
Fig.3 The β-sheet tertiary structure is energetically stabilized by interactions between hydrophobic patches (hatched) and intermolecular hydrogen bonding between the acetamido and carboxylate groups.6

