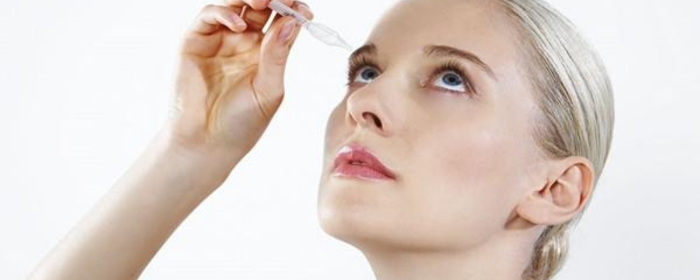
According to QYResearch, the global sodium hyaluronate (SH) eye drops market reached $309 million in sales in 2023. By 2030, it is expected to grow to $470 million, with a compound annual growth rate (CAGR) of 6.1%. This shows a steady growth trend in the global market for sodium hyaluronate eye drops.
What is Sodium Hyaluronate
Sodium hyaluronate (SH) is the salt form of hyaluronic acid (HA), originally isolated from the eye’s vitreous body. Research has shown that SH is widely distributed in animal and human tissues, particularly in the extracellular matrix. It is abundant in the vitreous body, aqueous humor, synovial fluid, skin, and umbilical cord.
Sodium hyaluronate is considered extremely viscoelastic, with unique rheological properties and pseudo plasticity. It does not possess cytotoxicity and holds excellent biocompatibility. These properties make it of great value in applications concerning ophthalmology and joint health.
Reference:
From Surgery to Daily Care: The Versatile Role of Sodium Hyaluronate in Eye Health
What is Sodium Hyaluronate Used for in Eye Drops
Sodium hyaluronate is one of the safest and most effective carriers in ophthalmic formulations. It relieves dry eyes and reduces drug-induced irritation, offering quick relief from discomfort.
1. Improves Drug Bioavailability
SH serves as a carrier in eye drop formulations, enhancing drug performance through its physical adhesion, membrane affinity, and drug-binding properties. These effects increase the bioavailability of drugs, reduce irritation, and keep the eyes hydrated and comfortable, promoting healing.
A study by Herrero et al. explored the effects of adding bioadhesive polymers, including SH, to a solution containing 2% tropicamide. The results showed that SH helped drugs remain on the eye surface longer, enabling more effective and sustained absorption by eye tissues.
2. Reduces Side Effects from Preservatives
Preservatives are very common ingredients in eye drops, and nearly all multi-dose packaged eye drops contain preservatives. On the other hand, long-term use of preservatives may cause injury to ocular surface cells. Fortunately, sodium hyaluronate has a powerful protective and antioxidant effect on the ocular surface, which can effectively reduce the adverse reactions caused by preservatives.
Its biocompatibility and lubricating properties form a protective film on the eye surface, preventing preservatives from directly contacting eye tissues and reducing toxicity. Additionally, SH’s high water-retention capacity dilutes preservatives, lowering their effective concentration and reducing irritation.
Molecular Weight of Hyaluronic Acid for Eye Drops Varies by Application
| Application | Molecular Weight | Characteristics |
| Daily Moisturizing | 800K ~ 1,500K Da | Moderate viscosity, good flowability; relieves mild dry eye by providing hydration and lubrication. |
| Dry Eye Treatment | 1,500K ~ 2,500K Da | Stronger hydration and adhesion; forms a stable protective layer for moderate to severe dry eye. |
| Post-Surgery Repair | 2,000K ~ 3,000K Da | High viscosity and extensibility; promotes eye surface repair and reduces post-surgery discomfort. |
| Anti-Fatigue | 1,000K ~ 1,800K Da | Medium viscosity; offers hydration and lubrication to relieve eye fatigue from prolonged use. |
| Drug Carrier | 500K ~ 1,200K Da | Lower molecular weight enhances drug delivery and absorption while maintaining light viscosity. |
Stanford Chemicals Company (SCC), a leading hyaluronic acid wholesaler based in the United States, offers a range of high, medium, and low molecular weight pure hyaluronic acid powders. Our naturally derived sodium hyaluronate powder is animal-free, vegan, and kosher-compliant.
| Item No. | Specification |
| HA-EM2.0-SC | M.W: 800K-1,300K Da, I.V.: 1.44-2.12 m3/kg |
| HA-EM2.4-SC | M.W: 1,300K-1,800K Da; I.V: 2.12-2.72 m3/kg |
| HA-EM3.0-SC | M.W:1,800K-2,500K Da; I.V.: 2.72-3.53 m3/kg |
| HA-EMC-SC | Customized Molecular weight |
